Law of conservation of mass
Summary of task
Prior to the sample task, students learnt the basic concepts and history of atomic theory, the difference between elements and compounds, the law of conservation of mass, and different types of chemical reactions.
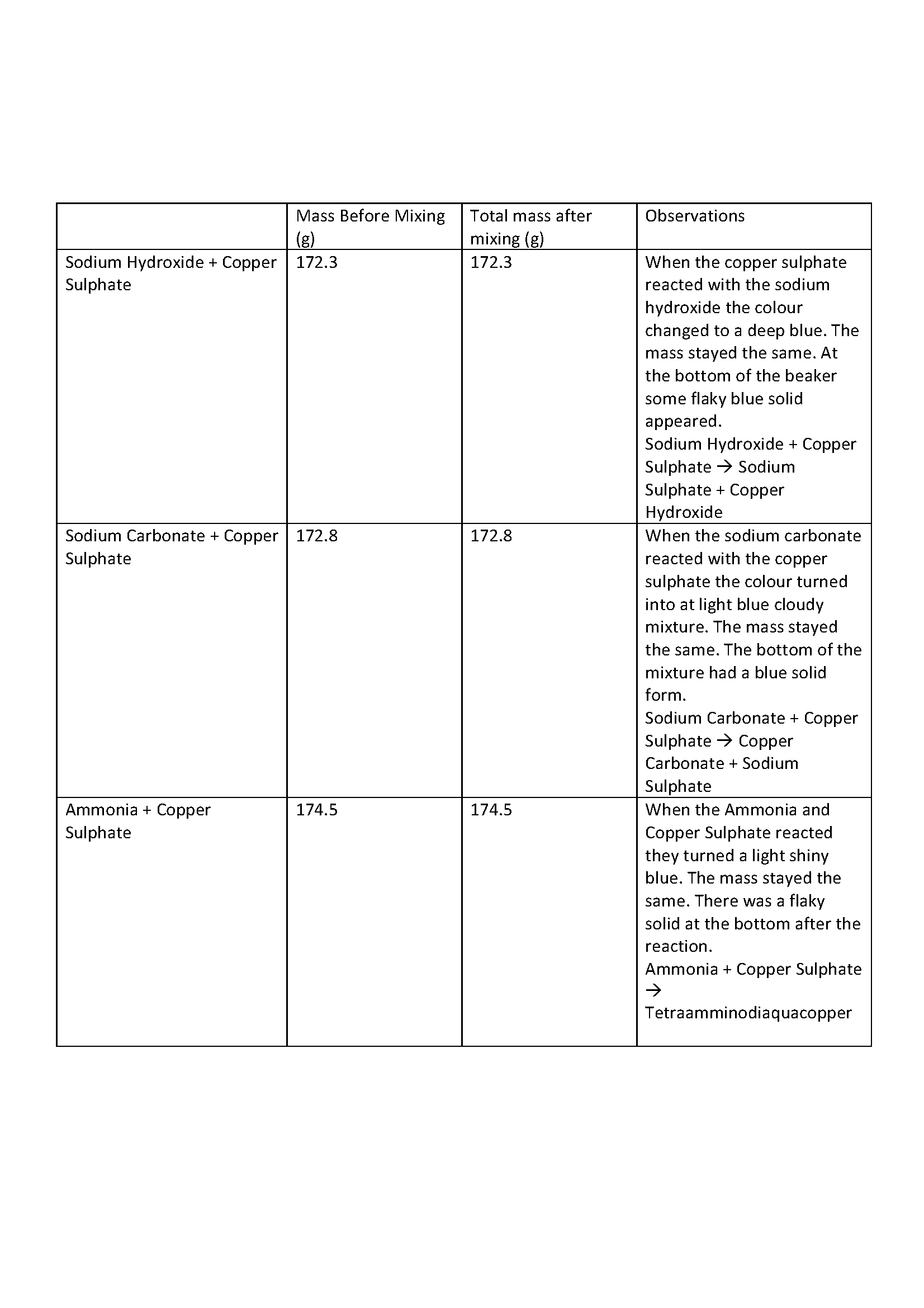
In this task, students were asked to perform three different chemical reactions to investigate the validity of the law of conservation of mass and to prepare a scientific report on their findings. Following a given procedure, students were asked to mix a solution of copper sulphate with solutions of sodium carbonate, sodium hydroxide, and ammonia. They were to record the mass of reactants before and after mixing and observe and record any changes that occurred. Students were provided with detailed guidelines on how to structure their report, what style of language to use and what content to cover in each section. In the introduction, students were asked to include:
- an explanation of chemical reactions
- three examples of chemical reactions (one helpful, one unhelpful and one used in industry) with explanations and balanced equations including state symbols
- a statement about the importance of the law of conservation of mass
- the reasoning behind carrying out the experiment
- the intended process and execution of the experiment.
In the discussion, students were asked to:
- list evidence that chemical reactions took place in all three reactions
- compare the total masses before and after mixing
- state the law of conservation of mass and assess whether the results support it
- explain whether they would have been able to demonstrate the law of conservation of mass to the same extent, if they had done a similar experiment using acetic acid and baking soda.
Students had two lessons for the practical work and four lessons to analyse their results and complete the report.